|
|
||||||||||||||
|
Abiraterone (Zytiga®) |

|
|||||||||||||
Abiraterone (Zytiga) is an established hormonal therapy for prostate cancer. The EU license was granted in September 2011. Abiraterone is now indicated for men with metastatic prostate cancer who have progressed on hormone therapy. In September 2012 the UK NICE committee rules it was cost effective and should be available on the NHS. Another drug in the same category is called enzalutamide. How it worksProstate cancer needs testosterone (male sex hormone) to grow. To make testosterone your body produces an enzyme called cytochrome p17. Abiraterone blocks cytochrome p17 and stops your body from making testosterone. This can slow down the growth of your cancer or shrink it. How you take abirateroneAbiraterone is available as tablets that you take once a day. The usual dose is 2 tablets or 4 tablets per day. You should swallow your tablets whole with a glass of water on an empty stomach. Take them at least one hour before food, or at least 2 hours afterwards. You take abiraterone with a steroid called prednisolone or dexamethasone to help reduce some of the side effects. You must take tablets and capsules according to the instructions your doctor or pharmacist gives you. Whether you have a full or empty stomach can affect how much of a drug gets into your bloodstream. You should take the right dose, not more or less. Talk to your specialist or advice line before you stop taking a cancer drug. If you forget to take a dose of abiraterone, prednisone or prednisolone, take your usual dose at the normal time the following day. If you forget to take abiraterone, prednisone or prednisolone for more than one day, talk to your doctor as soon as possible. Side effectsWe haven't listed all the side effects. It's very unlikely that you will have all of these side effects, but you might have some of them at the same time. How often and how severe the side effects are can vary from person to person. They also depend on what other treatments you're having. For example, your side effects could be worse if you're also having other drugs or radiotherapy. When to contact your teamYour doctor, nurse or pharmacist will go through the possible side effects. They will monitor you closely during treatment and check how you are at your appointments. Contact your advice line as soon as possible if:
Early treatment can help manage side effects better. Common side effectsThese side effects happen in more than 10 in 100 people (10%). You might have one or more of them. They include: Fluid build up (oedema)You may have swelling of your hands and legs due to a build up of fluid (oedema). Urinary tract infectionTalk to the team looking after you about this. Increased blood pressureTell your doctor or nurse if you have headaches, nose bleeds, blurred or double vision or shortness of breath. Your nurse will check your blood pressure regularly. DiarrhoeaContact your advice line if you have diarrhoea, such as if you've had 4 or more loose watery poos (stools) in 24 hours. Or if you can't drink to replace the lost fluid. Or if it carries on for more than 3 days. Your doctor may give you anti diarrhoea medicine to take home with you after treatment. Eat less fibre, avoid raw fruits, fruit juice, cereals and vegetables, and drink plenty to replace the fluid lost. Low potassium levels in the bloodYou will have regular blood tests to check for this. Occasional side effectsThese side effects happen in between 1 and 10 out of every 100 people (1 to 10%). You might have one or more of them. They include:
Rare side effectsThese side effects happen in fewer than 1 in 100 people (1%). You might have one or more of them. They include:
Contraception and drug handlingThis drug may have a harmful effect on a developing baby. It is important not to father a child during treatment. Talk to your doctor or nurse about contraception before having treatment if there is any chance that your partner could become pregnant. You need to use a condom and another effective birth control method. If you have sex with a pregnant woman, use a condom to protect the unborn child. Abiraterone is not for use in women and can cause harm to the unborn child if it is taken by women who are pregnant. Women who are pregnant or who may be pregnant should wear gloves if they need to touch or handle abiraterone tablets. Lactose, sodium and abirateroneAbiraterone contains a type of sugar called lactose. If you have an intolerance to some sugars, contact your doctor before taking this medicine. Abiraterone also contains some salt (sodium). Talk to your doctor before starting treatment if you are on a controlled sodium diet. Background evidence The results of a UK trial of abiraterone have been published recently involving men with advanced metastatic advanced prostate cancer. This was a phase 3 study to comparing the clinical benefit of abiraterone acetate plus prednisolone with placebo plus prednisolone in patients with metastatic castration-resistant prostate cancer. It was published in the Journal of Clinical Oncology and involved men with prostate cancer (CRPC) who have already received hormones for their disease failed one or two chemotherapy regimens. At least one of the previous chemotherapies must have contained docetaxel. An update of this trial was also published in the ESMO conference Milan 2010. The results suggest that abiraterone will be a useful addition to the available drugs for Hormone refractory prostate cancer. How does abiraterone work? It blocks the formation of the male hormone testosterone by inhibiting an enzyme called CYP450c17, also known as 17α-hydroxylase/17,20 lyase. This enzyme is involved in the formation of DHEA, which ultimately is metabolized into testosterone. The Primary Outcome Measure of the trial was overall survival and the secondary outcome measures was the proportion of patients achieving a PSA decliine ≥ 50% according to Prostate Specific Antigen Working Group (PSAWG) criteria. Men in this and another similar study were given 1000mg of abiraterone daily. The results showed that in this category of disease abiraterone lowered PSA, it shrank the size of tumours and doubled their rate of survival. (in effect about by about 3 months). These are promising results but further tests are necessary. Only through well conducted research will we be able to truly know the full benefit of this drug. Cougar Biotechnology Inc. of Los Angeles, the company which owns abiraterone, expects the drug to reach the market in 2011. In the mean time it is only available in a trial setting. (About clinical trails) Information on the latest Abiraterone trial presented in ESMO 2010 This study included 1,195 patients with metastatic advanced prostate cancer (castration-resistant prostate cancer, or CRPC) previously treated with one or two chemotherapy regimens, at least one of which contained docetaxel. The results demonstrate that patients treated with abiraterone acetate plus low dose prednisone/prednisolone showed a significant improvement in overall survival compared to patients treated with prednisone/prednisolone plus placebo – specifically: Treatment with abiraterone acetate resulted in a 35 percent reduction in the risk of death (HR=0.65; 95 percent CI: 0.54, 0.77; p<0.0001) and a 36 percent increase in median survival (14.8 months vs. 10.9 months) compared with placebo. Patients
who received abiraterone acetate and low dose prednisone/prednisolone also
showed significant improvements in secondary study endpoints when compared to
the prednisone/prednisolone plus placebo group: time to PSA progression (TTPP)
[median 10.2 months for abiraterone acetate vs. 6.6 months for placebo,
HR=0.58 (95 percent CI: 0.46, 0.73); p<0.0001] and an increase in
radiographic progression free survival (rPFS) [median 5.6 months for
abiraterone acetate vs. 3.6 months for placebo, HR=0.67 (95 percent CI: 0.58,
0.78); p<0.0001]. Total PSA response, defined as greater than or
equal to a 50 percent decrease from baseline, was achieved in 38 percent of
patients treated with abiraterone acetate vs. 10 percent in the prednisone/prednisolone
plus placebo group [p<0.0001]. Patients in the abiraterone acetate group experienced more mineralocorticoid-related adverse events than those in the prednisone/prednisolone plus placebo group. The most frequent adverse events were fluid retention (30.5 percent vs. 22.3 percent) and hypokalemia (17.1 percent vs. 8.4 percent). Grade 3/4 hypokalemia and hypertension were more frequent in the abiraterone acetate arm than in the placebo arm (3.8 percent vs. 0.8 percent and 1.3 percent vs. 0.3 percent, respectively). Liver function test abnormalities were observed in 10.4 percent of abiraterone acetate treated patients compared to 8.1 percent in the prednisone/prednisolone plus placebo group. Cardiac disorders were observed in 12.5 percent of abiraterone acetate patients vs. 9.4 percent of patients who received placebo. Mechanism-based adverse events were amenable to medical management and distinct from adverse events commonly associated with cytotoxic chemotherapy. This randomized, double-blind placebo-controlled Phase 3 study was conducted in 147 centers in 13 countries. Patients with metastatic advanced prostate cancer previously treated with docetaxel (N=1,195) were randomly assigned 2:1 to receive abiraterone acetate (1000 mg once daily) plus prednisone/prednisolone (5 mg twice daily) (N = 797), or placebo plus prednisone/prednisolone (N = 398). The primary endpoint was overall survival. More about prostate cancer (Prostate cancer UK clinical guidelines): UK
incidence: The risk of this condition increases with the age, generally men over the age of 50 are
affected. In the UK nearly
32,000 men are diagnosed every year with prostate cancer which causes more than
10,000 deaths every year. Overall, one men in fourteen will get prostate cancer
at some point in his life.
Types: Although there are several cell types in the prostate, nearly
all prostate cancers start in the gland cells. This kind of malignancy is known
as adenocarcinoma (find out more). Risks factors: Most cases of this condition are diagnosed in men over
the age of 65. There are usually no predisposing risk factors but there is some evidence
of associations with a previous poor diet (high in fat, low in fruits and
vegetables), being a black male, men who have a strong family history of the disease are at greater
risk. (find out more) Tests to determine a diagnosis and help decide on treatment options: Prostate-Specific
Antigen (PSA) blood test, Digital Rectal Examination (DRE), transrectal
ultrasound (TRUS), biopsy (find out more) What determines the treatment options and prognosis: Treatment options (find
out more): Prostate cancer can often grow very slowly and most men with this
diagnosis live their lives without it causing any problems to them. Therefore
there is a lot to consider when deciding the best way to treat prostate cancer.
It may take more than one visit to your doctor to discuss all your concerns and
you may want to consider getting a second opinion. Make sure all your concerns
and questions have been answered before you decide on a treatment. Your
treatment plan may involve one or a combination of treatments: |
||||||||||||||
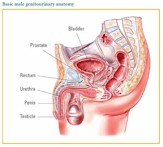 Presenting
symptoms: Possible symptoms of prostate cancer include blood in the urine or
semen, pain and trouble passing urine, frequent urination
Presenting
symptoms: Possible symptoms of prostate cancer include blood in the urine or
semen, pain and trouble passing urine, frequent urination