A double blind, randomised evaluation of a polyphenol rich natural nail bed balm to prevent chemotherapy-induced damage
.Summary:
This UK study, designed with the National Cancer Research Institutes (NCRI) clinical trials development committee, aims to find a way to help reduce the incidence and severity of nail damage associated with chemotherapy.
See
full published paper
| Link
to Polybalm website
| Links to nail
health guidelines
Background
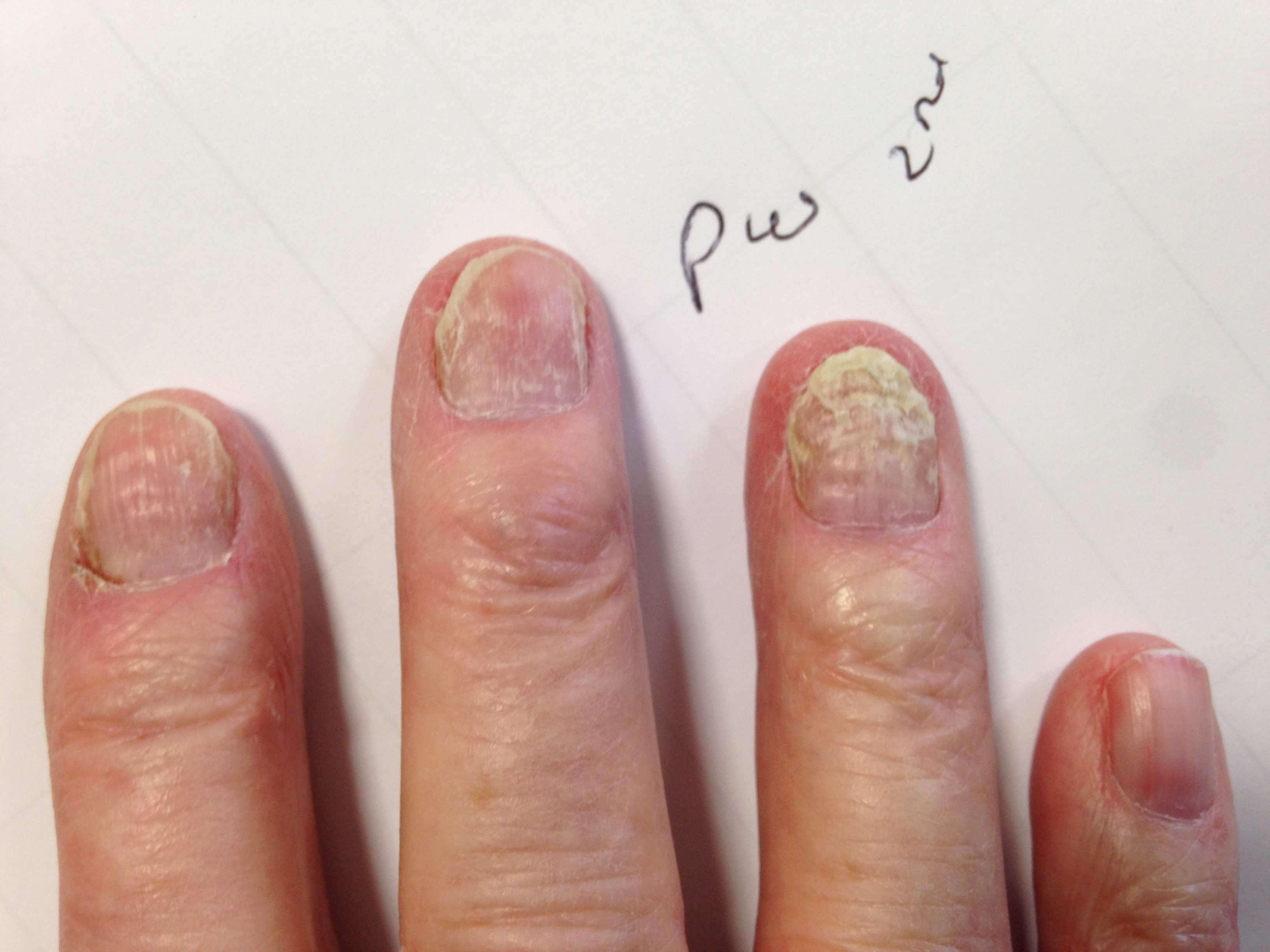 Disfiguring nail damage is common after
chemotherapy, especially the taxane category, often used in the treatment of breast, prostate and lung cancer. Nail damage can be unsightly and contributes to body image issues but also to more serious consequences include pain, which can limit activities of daily living, and lead secondary fungal and bacterial infections
1,2. Disfiguring nail damage is common after
chemotherapy, especially the taxane category, often used in the treatment of breast, prostate and lung cancer. Nail damage can be unsightly and contributes to body image issues but also to more serious consequences include pain, which can limit activities of daily living, and lead secondary fungal and bacterial infections
1,2.
We have previously published a report showing that cooling the nails bed with pots of iced water helps to reduce its
severity4. Understandably, the practice has never caught on within the confines of a busy chemotherapy unit. Some commercially available cooling gloves are available but they are not particularly popular among UK chemotherapy nurses as they may cover the veins of the hands and prevent assessment of the
patient's extremities7.
Nails are made by living cells in the nail beds which, because they are dividing rapidly, are especially susceptible to chemotherapy. Particular sensitivity to taxotane chemotherapy is thought to be due to their anti-angiogenetic and neurogenically mediated inflammatory
properties3,4.
Patient advocacy groups advise wearing nail varnish, massaging the nail bed with a balm to maintain moisture, prevent skin splitting in the hope it reduces damage to the nails. There are no published prospective trials to support the practice use during chemotherapy, or in an otherwise healthy population. Likewise, there is no guidance on the type of balm to use.
How could the ingredients of this balm help the nails - The
hypothesis?
Oiling skin around the cuticles moisturizes and improves the compliance of the skin, preventing splitting which can be a route for infection and hence further damage to the nail beds. Using natural oil bases, rather than some synthetic creams, avoids chemicals such as perfumes, parabens, preservatives or colours, which may be irritating. The natural bases and essential oils were selected for their anti-inflammatory, anti-oxidant and anti-microbial properties.
Study
design
This is a double blind, randomised trial involving 60 patients receiving chemotherapy. Half will receive the polyphenol rich balm and the other half a simple petroleum based moisturiser. The study has been designed in this way to prevent biased results; neither the participant nor the research team (including your doctor), will know which of the creams is the natural wax-based nail cream being tested, and which is the petroleum-based cream, until after the study has finished. All patients has started cytotoxic chemotherapy (or about to start within 2 weeks), at least 50 receiving taxanes (taxotere or taxol). Participants are asked to massage a small amount onto the nails beds three times a day. Assessment of nail damage were taken at the start and at the end of the study (3 weeks after the last injection of chemotherapy).
What is
measured - trial end points?
-
A nail health questionnaire, completed by the participant at the start and end of the intervention. (Dermatology Life Quality Index)
-
A linear analogue scale of the participants satisfaction with their nails at the start and end of the study
-
Photographs of the nail nails of the hands at the start and end of the study ? then toxicity scoring of the visual health of the nails by three independent doctors
(NAPSI score and Common Toxicity Criteria score)
(see full questionnaires)
Quality assurance
The scientific trial team included cancer doctors (0ncologist), skin doctors (dermatologists), nurses, statisticians as well as patients themselves. All the medical staff involved in the study had Good Clinical Practice (GCP) research training.
It was designed with the help of the independent government-funded body - The National Cancer Research clinical trials development team which ensured it had the highest possible scientific structure. It was audited by an external agency to ensure full compliance and approved by the Cambridge Central Research Ethics Committee.
The active balm and identically looking and smelling placebo were made by an independent supplier of cosmetics in the UK. Labelled with either ?A? or ?B? and sent directed to the trials unit who were blind to which was which. Sixty opaque envelopes contained either 30 cards reading
"Cream A" or reading "Cream B". Envelopes were sealed with sealing wax
molded to the opening of the envelope, to ensure that they cannot be opened without breaking the seal. The cards are then shuffled, labelled 1-60 and placed in a box. The card envelopes were then removed and opened in strict numerical order.
All data was stored on a password-protected Bedford Hospital NHS Trust computer. Once the data has been completed, the database will be ?locked? in a password-protected file. The database will be sent for independent statistical analysis to Michael Cauchi from Cranfield University, Bedfordshire. He will not know which patients received the active cream and which placebo when he analyses the data ? a further ?blinded? assurance.
Statistical considerations
Data and efficacy endpoints will be summarised using descriptive statistics (number of observations, mean, standard deviation, median, minimum and maximum). For endpoints subjected to formal statistical analysis, the results of the analysis will be summarised and 95% confidence intervals for treatment differences will be presented and displayed as box-and-whisker plots which would also indicate the variability in the data. All efficacy variables will be analysed for the intention-to-treat population. No adjustment for multiple comparisons will be made. The photographs will be stored digitally with the future intent of subjecting to multivariate data analysis in conjunction with image processing in order to correlate the human observations with the machine learning algorithms.
Sample size and power considerations. The incidence of chemotherapy-induced onycholysis is between
40-70%2,7. A drop in the toxicity score by 20% (1 of 5 in a Likert scale) is deemed to be clinically significant, by the trial development committee. There are no previous trials of nail bed balms to guide the power calculation. The two published studies using similar toxicity grading scores, evaluating the benefits of a frozen glove on the incidence of onycholysis had 20 and 25 patients respectively to produce an 80% statistical power for a two-sided test at the
à=0.05
level1,7.
The blind nature of the study and randomisation confers a more powerful statistical model than these studies. We estimate that 50 patients will more than adequately detect a 20% difference in toxicity with a
û2*sd of confidence of using a two-sided test at the à=0.05 level.
A further 10 patients were added to improve statsitical power and cover for any
early drop outs.
Contact
information
Independent advice or further information on participating in a clinical trial in general you can contact: Macmillan Cancer Support on 0808 808 0000, or visit their website www.macmillan.org.uk; The National Institute for Health Research (NIHR), email: oktoask@nihr.ac.uk, or visit their website www.nihr.ac.uk The local research manager lead is Madeleine Williams, Tel: 01234 795787. Email:
madeleine.williams@bedfordhospital.nhs.uk The
chief investigator for this trial is Dr Sarah Smith, Consultant Oncologits
Bedford and Addenbrooke's Cambridge University NHS Trusts.
 Post trial
information Post trial
information
The abstract of
the final trial data was presented at ASCO 2017 - see
results. The product polybalm is now made
by an UK manufacturer which has no connection with the trials unit which ensures
its availability nationally and internationally.
Read
more about Polybalm - the product
References:
-
Ishiguro et al: Freezing with gloves for prevention of docetaxel-induced nail toxicity in breast cancer patients. Support Care Cancer 20:2017-2024, 2012
-
Minisini AM, Tosti A, Sobrero AF, et al: Taxane-induced nail changes: Incidence, clinical presentation and outcome. Ann Oncol 14:333-337, 2003
-
Battegay EJ: Angiogenesis: Mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med 73:333-346, 1995
-
Wasner G, Hilpert F, Schattschneider J, et al: Docetaxel-in nail changes: A neurogenic mechanism?A case report. J Neurooncol 58:167-174, 2002
-
Ding PN, Thomas RJ: A cool solution for docetaxel induced onycholysis. Clinical Focus on Cancer Medicine 2(1):18-19, 2010
-
Katsimbri P, Bamias A, Pavlidis N: Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36:766-771, 2000
-
Scottie F et al: Muticenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity. J Clinical Oncology 23 (19): 4424-4429, 2005
-
Milin C, Domitrovi R, Tota J, et al: Effect of olive and corn oil enriched diets on the tissue mineral content in mice. Biol Trace Elem Res 82(1-3): 201-210, 2001
-
Purba MB, Kouris-Blazos A, Wattanapenpaiboon N, et al: Skin wrinkling: can food make a difference? J Am Coll Nutr 20: 71-80, 2001
-
Ichihashia M, et al: Preventive effect of antioxidant on ultraviolet-induced skin cancer in mice. Journal of Dermatological Science
23:S45S50, 2000 (Supp. 1)
-
Federal Register (2003). Skin protectant drug products for over-the-counter human use: final monograph.68:33362-33381.
-
Jensen CD, Andersen KE: Allergic contact dermatitis from cera alba (purified propolis) in a balms. Contact Dermatitis 55:312-313, 1999
-
Rogers SR, Bekic M: Diseases of the lips. Seminars in Cutaneous Medicine and Surgery 16:328-336, 1997
-
Pray WS: Treatment of Chapped Lips (Cheilitis). US Pharm 5:68-69, 2005
-
Delaquis PJ, Stanich K, Girard B, et al: Antimicrobial activity of individual of essential oils. Int J Food Microbiol 74(1-2):101-109, 2002
-
Smith-Palmer A et al: Antimicrobial properties of plant essential oils and essences against
food-borne pathogens. Applied Microbiology 26(2):118-122, 2002
-
Baratta MT et al: Antimicrobial and antioxidant properties of some commercial essential oils. Flavour and Fragrance Journal 13(4):235-244, 2001
-
Thomas R et al: Giving patients a choice improves quality of life: A multi-centre, Investigator-blind, randomised, crossover
study Clin Oncol (2004) 16: 485-49.
-
Cassell S, et. al.: The Modified Nail Psoriasis Severity Index: Validation of an Instrument of Assess Psoriatic Nail Involvement
score. J Rheumatol 2007;34:123-9
-
Rich P and Scher RK: Nail Psoriasis Severity Index: A useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003;33:1452-6
-
CTCAE 4.03 - June 14, 2010: Skin and subcutaneous tissue disorders. National Cancer Institute. NIH Publication No. 09-5410.
|

 Disfiguring nail damage is common after
Disfiguring nail damage is common after